“An arrow deemed retrosynthetic
Portrays a reflective aesthetic:
Reverse contemplation
To starting location
In pondering routes hypothetic.”
The next type of reaction arrow highlighted in verse, in the 19 April 2023 Twitter limerick, was the “retrosynthetic arrow,” which visually indicates a specific type of analysis most typically completed by organic chemists.
“An arrow deemed retrosynthetic /
Portrays a reflective aesthetic…”
The retrosynthesis arrow has one of the most distinctive appearances of the arrows described in these poems, indicating the need for retrosynthesis: thinking backwards, via a “reflective aesthetic.” It is a hollow arrow pointing from the left to the right. It is probably most illustrative to contrast it with the other left-to-right arrow we’ve seen thus far.
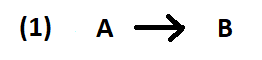
Reaction 1 (above) is the typical case we’ve seen before: Reactant A forms Product B.
By contrast, Reaction 2 below indicates that we consider Species A as the target molecule in an organic synthetic project (the molecule that we want to synthesize in the lab). Species B in this case is the precursor: something that could be used as a reactant to form target Species A as a product. The unusual arrow communicates this thought process.
“Reverse contemplation /
To starting location…”
Typically, then, we would continue thinking backwards, as in simplified Reaction 3: all the way to starting materials that were readily available (either easy-to-synthesize or listed in a catalog), generalized here as Species D.
In poetic terms, our “reverse contemplation” would continue all the way back to “starting location.”
“In pondering routes hypothetic.”
Chemist E. J. Corey won the Nobel Prize in 1990 for formally developing the technique of retrosynthesis. This “pondering [of] routes hypothetic” has led to many insights in organic chemistry.
Here, the subtle difference in arrow notation indicates a specific and valuable technique for an organic chemist to understand.
You must be logged in to post a comment.